
Pacran’s™ E. coli reduction of adhesion activity was established in multiple ex vivo studies.⁴ ⁷⁻¹⁰ A clinical study demonstrated that Pacran™ has the ability to lower urinary E. coli load, which is one of the mechanisms of action to reduce symptoms in women with active UTIs.⁸
One clinical study looked specifically at women with a history of urinary tract infections. Among those who consumed Pacran™ for six months, there was a reduction in UTI incidence, longer time to first UTI, and reduced average number of UTIs compared to those who were given a placebo.⁹
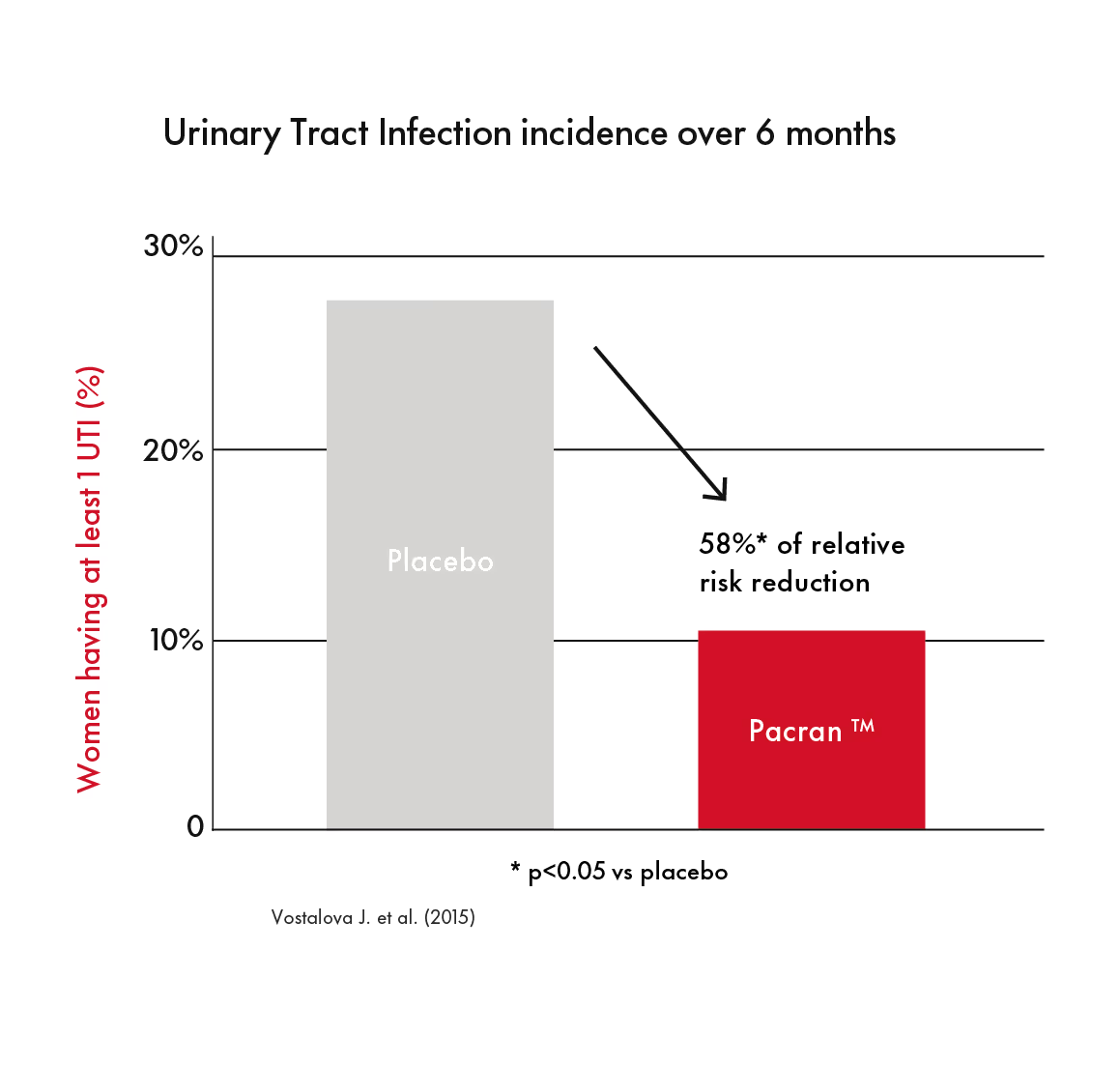
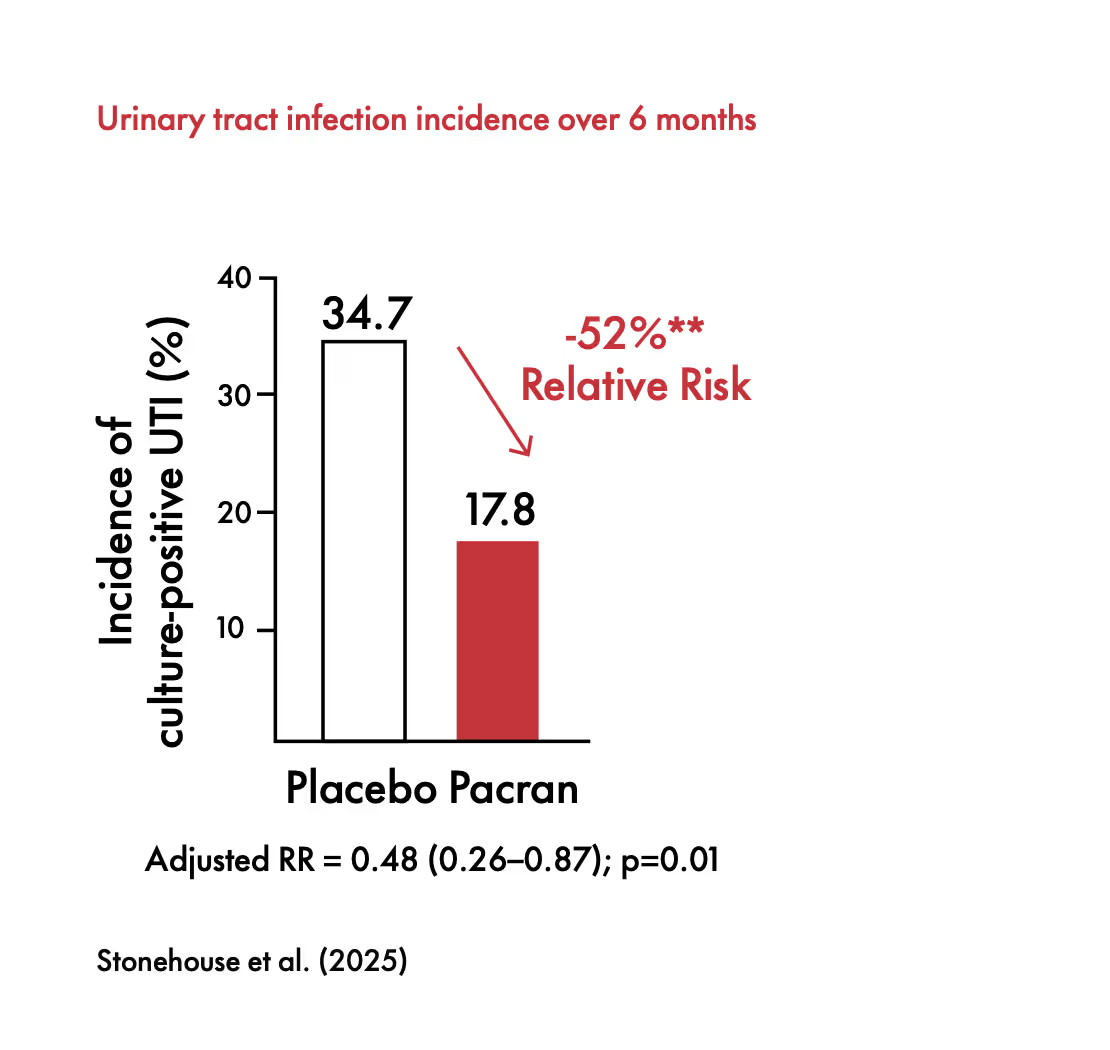
Pacran™ was studied in a population of women with a history of recurrent UTI (defined as ≥2 acute UTIs within 6 months or at least 3 within a year). Pacran™ consumption led to a significant reduction in culture-confirmed UTIs, longer time to first culture positive UTI, reduction in average number of UTI. Additionally, this study was the first Pacran™ study to show reduction in UTI incidence with urinary frequency and urgency symptoms.¹⁰
Together, these two studies on women with a history of UTI offer compelling evidence that the ingredient may not only reduce recurrent UTI but also provide relief from the discomfort often associated with uncomfortable symptoms of UTI, such as; urinary frequency and urinary urgency.¹³ ¹⁴
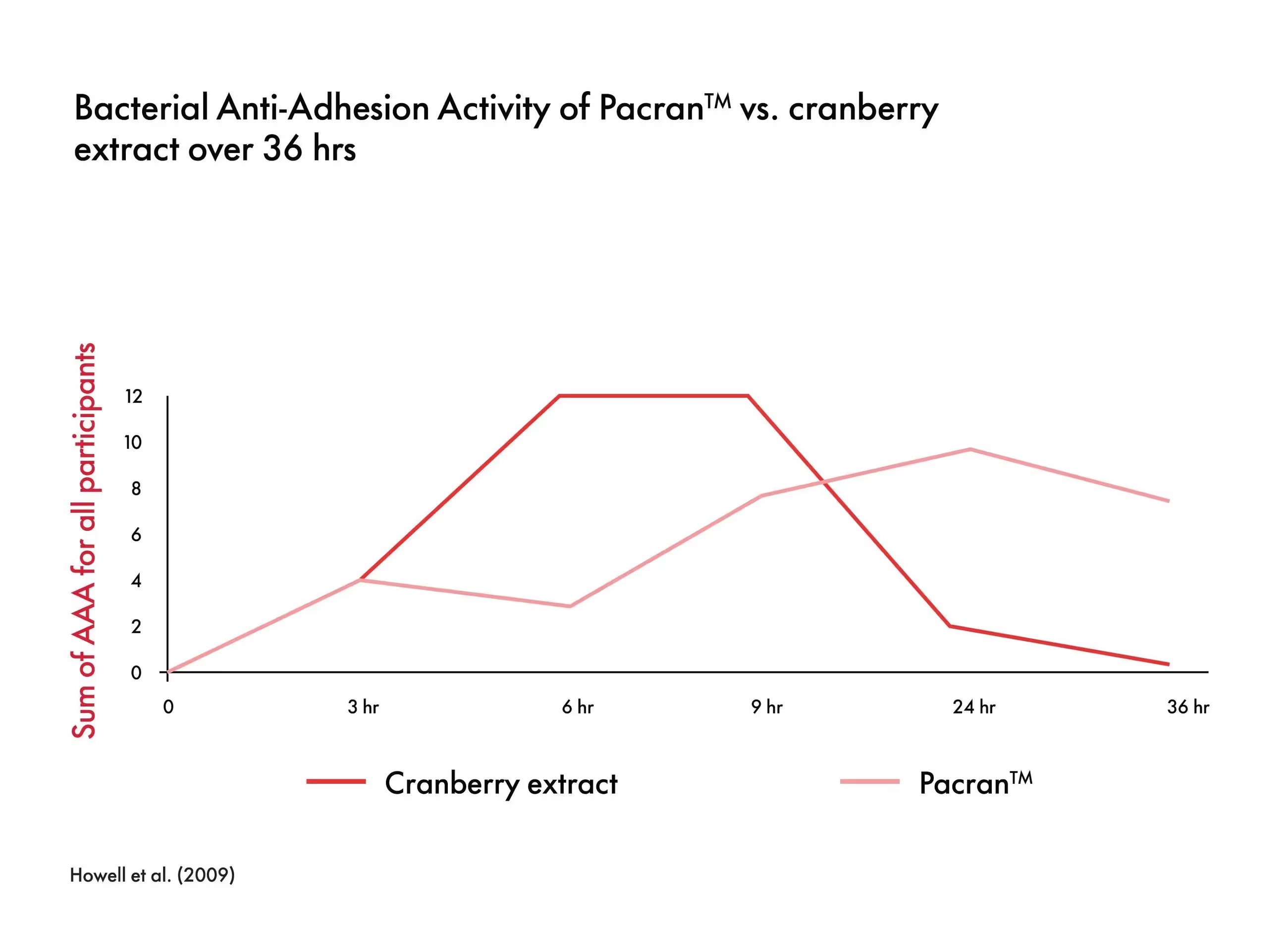
Many competitive cranberry ingredients are built to deliver the maximum amount of cranberry-PACs. These ingredients are quite expensive on a dosage basis as they require multi-stage extraction processes and a costly biomass for extraction. Pacran™ has been shown in an ex vivo study to deliver an E. coli reduction of adhesion activity, similar to cranberry extracts with 20 times more PACs in the short term (0-9 hours) and superior in the longer term (9-36 hours).⁴ These data suggest cranberry based urinary tract health isn’t just about PACs but rather about delivering the right blend of the whole cranberry Pacran™.⁴
Just 500 mg per day of PacranTM has been shown to be sufficient to achieve a positive effect. Furthermore, it delivers similar reduction of adhesion activity to alternatives with a PAC content 20 times higher. This _ex vivo _research also shows that Pacran’sTM reduction of adhesion activity acts rapidly (anti-adhesion), reaching significance in nine hours, and peaking at 24 hours after consumption.⁴

2009: Pacran™ was awarded the country’s first-ever health claim for a branded cranberry ingredient with the claim: “May help to maintain urinary tract health by preventing urinary tract infection.”¹¹
2014: Health Canada granted Pacran™ a National Product Number authorising claims including “Helps prevent recurrent urinary tract infections (UTIs) in women or used in Herbal Medicine to help prevent recurrent urinary tract infections (UTIs).”¹²
2015: The Colombian authorities granted Pacran™ the claim: “Helps in the management of recurrent UTI.”¹³
2020: the US FDA published a Qualified Health Claim stating the benefits of whole cranberry powder consumption in reducing risk of UTI in healthy women. Our peer-reviewed publication on Pacran™ (Vostalova et al) was one of only three studies supporting the claim and was classed by the FDA as being of high methodological quality.¹⁴

